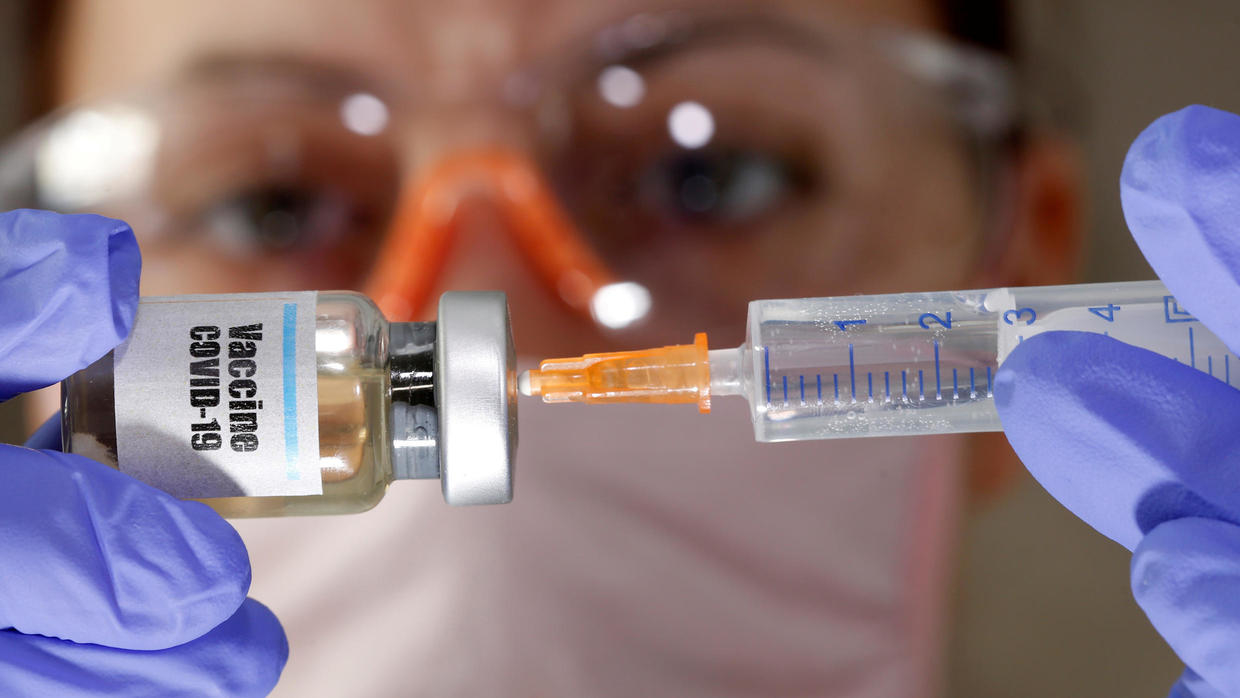
As efforts to find an effective vaccine for SARS-CoV-2 ramp up, an immunologist warns that fast-tracking of clinical trials could be catastrophic.
All data and statistics are based on publicly available data at the time of publication. Some information may be out of date.
Scientists around the globe are working to develop an effective vaccine for the novel coronavirus, SARS-CoV-2.
Until such a time, the world is reliant on physical distancing measures and personal protective equipment (PPE). Some countries are using ‘track and trace’ systems to monitor the movements of people and notify those who have been in contact with somebody diagnosed with COVID-19.
Of course, a treatment for COVID-19 is also highly desirable. Gilead’s anti-viral drug remdesivir appears to accelerate recovery time in some people. The United States and the United Kingdom have now authorized its use as a treatment for people with COVID-19.
However, as remdesivir is a treatment, not a cure, and some clinical trial results show no significant benefit, a vaccine remains preferable.
Although a COVID-19 vaccine is urgent, scientists cannot rush the development process. In a recent editorial, immunologist and deputy editor of the journal Science Advances Dr. Douglas J. Green explains why bypassing essential clinical trial stages for any such vaccine could be ‘catastrophic.’
Some experts have also advocated for ‘fast-tracking’ some clinical trial stages. They suggest that evidence that a vaccine triggers the production of neutralizing antibodies could be sufficient to progress to widespread implementation.
The article in Science Advances warns that this route could be dangerous and says scientists must conduct comprehensive safety tests for any potential vaccine.
Alex
Koordynator projektu