Promising early results from Chinese vaccine trials
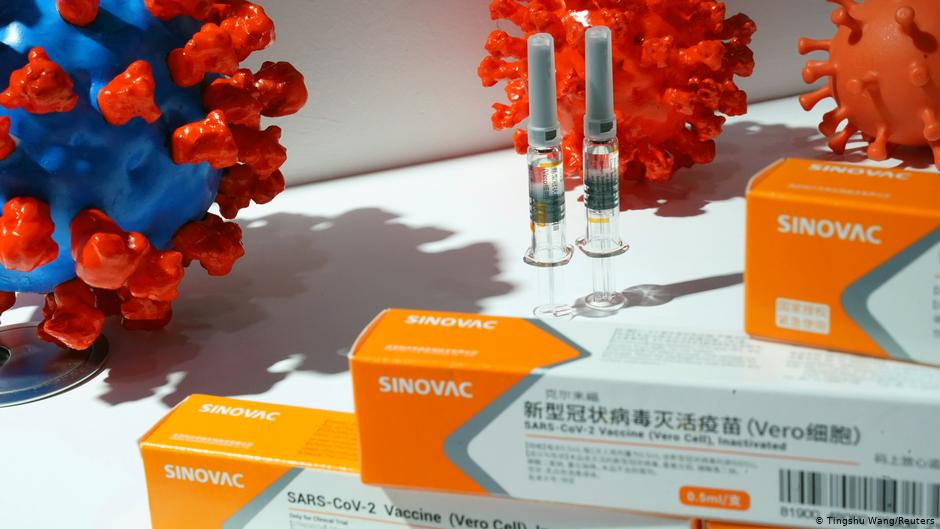
Yesterday, The Lancet Infectious Diseases published the results of a Phase 1/2 clinical trial on CoronaVac, a vaccine candidate designed in China. The study included more than 700 participants, aged 18–59. CoronaVac appears to be safe and elicit an immune response.
The researchers gave theparticipants two doses of the vaccine 14 days apart. Within 28 days of the first dose, they detected robust antibody responses.
However, this study was not designed to test how effective the vaccine is. Rather, it assessed the immune response and its safety. Ongoing phase 3 trials are investigating CoronaVac’s efficacy.
The results leave certain questions unanswered; for instance, the recent study did not assess T-cell activity. The authors are addressing this in the phase 3 trials.
Similarly, scientists will need to carry out further research to understand whether the vaccine is effective over the longer term. Additionally, the recent study only recruited healthy adults without existing health conditions.
“CoronaVac is one of many COVID-19 vaccine candidates that are being explored in parallel. There are a multitude of different vaccine technologies under investigation, each with their own advantages and disadvantages,” explains one of the authors, Dr. Gang Zeng from Sinovac Biotech.
He continues: “CoronaVac could be an attractive option because it can be stored in a standard refrigerator between 2°C and 8°C [35.6°F–46.4°F], which is typical for many existing vaccines, including [that for the] flu. The vaccine may also remain stable for up to 3 years in storage, which would offer some advantages for distribution to regions where access to refrigeration is challenging. However, data from phase 3 studies will be crucial before any recommendations about the potential uses of CoronaVac can be made.”